 June 1, 2024- by Steven E. Greer, MD
June 1, 2024- by Steven E. Greer, MD
Timed to have the news obscured by a Friday release and the Trump circus, the subsidiary of the military called Moderna received “FDA approval” for another mRNA gene therapy. This one is for RSV, which is a respiratory virus that is not lethal in almost all cases.
This rapid approval was awarded despite no adequate safety follow-up (i.e., only 3.7 months). But we know from the COVID mRNA gene therapies, which substituted the nucleoside uridine (U) with pseudouridine (Ψ)g to make the mRNA stable, that this modification resulted in gibberish protein production that can lead to all sorts of adverse events.
However, the junk-science paper in the NEJM stated:
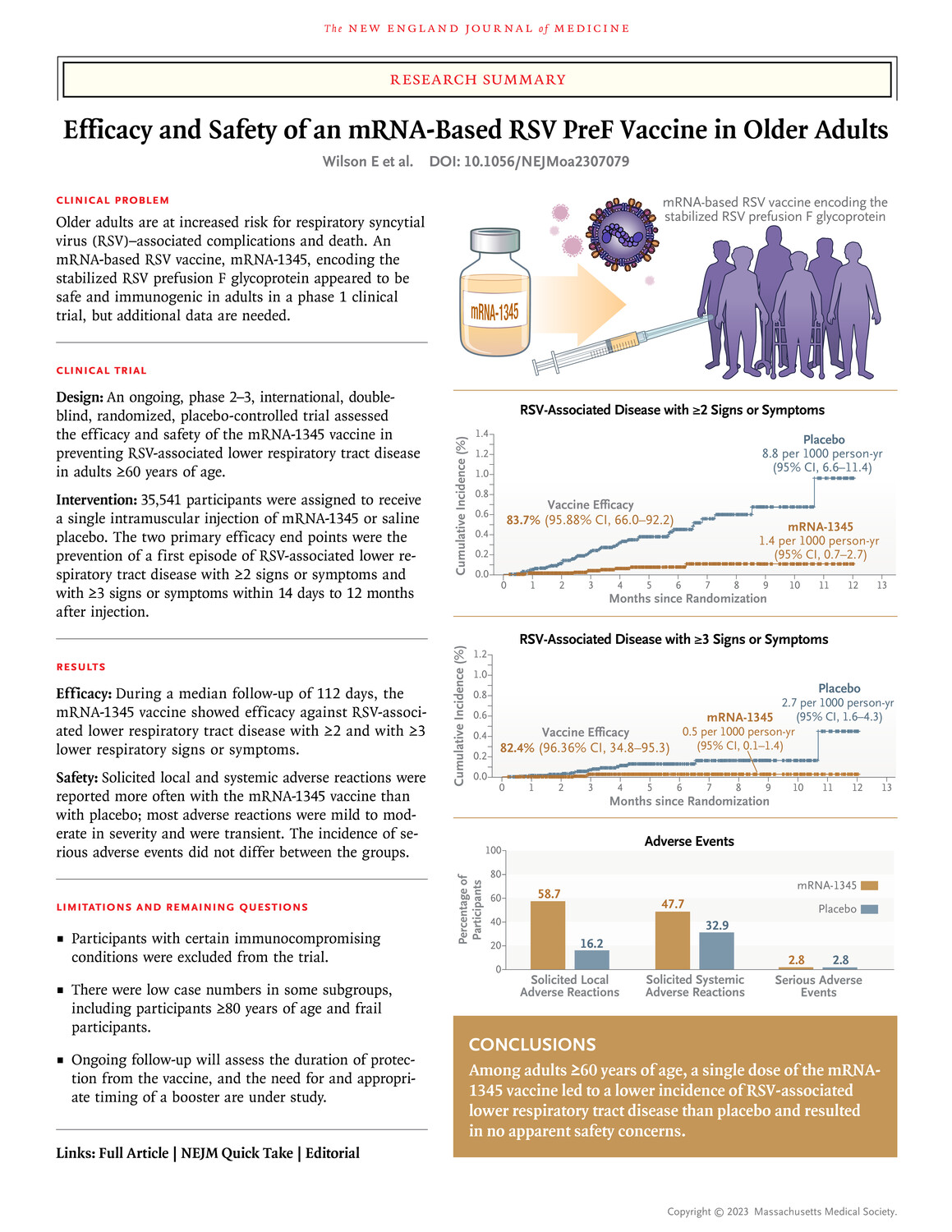
“Safety: Solicited local and systemic adverse reactions were reported more often with the RNA-1345 vaccine than with placebo; most adverse reactions were mild to moderate in severity and were transient. The incidence of serious adverse events did not differ between the groups.”, but the Moderna press release stated, “No serious safety concerns were identified in the Phase 3 trial.”
Normally, the NEJM would make the PDF of such an important trial free to the public. This time, it is behind a pay wall.
What exactly were those “mild” and “transient” adverse events. Were they like the “rare” and “transient” heart damage and cancer from the COVID shots? Why was such an unimportant drug approved without years of safety follow-up, especially given the proven harms of the COVID shots?
How about efficacy? The end-points were clinically meaningless. Oh, by the way, the company is already talking about the need for booster shots.
Clearly, the military and other deep state agencies are using the low bar set by the COVID fearmongering to now get other mRNA gene therapies approved as part of their furtherance of bioweapons programs. These so-called “vaccines” can be changed in a lab quickly and produced rapidly, which is required for a bioweapons program.
